Multiple Myeloma
CAR T-cell Therapy
Chimeric antigen receptor (CAR) T-cell therapy is a form of immunotherapy that involves modifying T-cells to recognize and kill cancer cells. Although CAR T-cell therapy has been previously available to treat some types of lymphoma and leukemia, this treatment option is now newly approved for treating relapsed and refractory multiple myeloma.
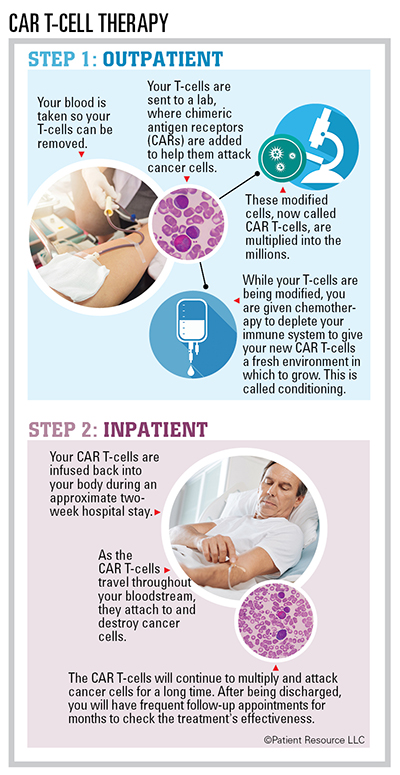
The CAR T-cell therapy process is autologous, meaning it uses only the patient’s T-cells. Donor cells are not involved.
CAR T-cell therapy was approved after clinical trials resulted in partial remission in the majority of multiple myeloma clinical trial participants. Many also experienced a lengthy response duration. Another benefit is that the CAR T-cells may remain in the body for months after treatment ends, continuing to attack cancer cells.
As with most cancer treatments, however, CAR T-cell therapy has potential risks of side effects (see Supportive Care). Your medical team will inform you about what to watch for so you are aware of when to seek help. You are encouraged to carry a Medical I.D. card with you in case of emergency so any treating physician is aware that you are taking immunotherapy and are susceptible to very serious side effects. Print a blank card at www.PatientResource.com/Wallet_Card.
At this time, multiple lines of therapy must be tried before CAR T-cell therapy can be used. However, promising clinical trial results offer hope that the therapy may eventually be used earlier in the treatment process. Ask your doctor if you should consider CAR T-cell therapy.



