Clinical Trials
Searching for a Clinical Trial
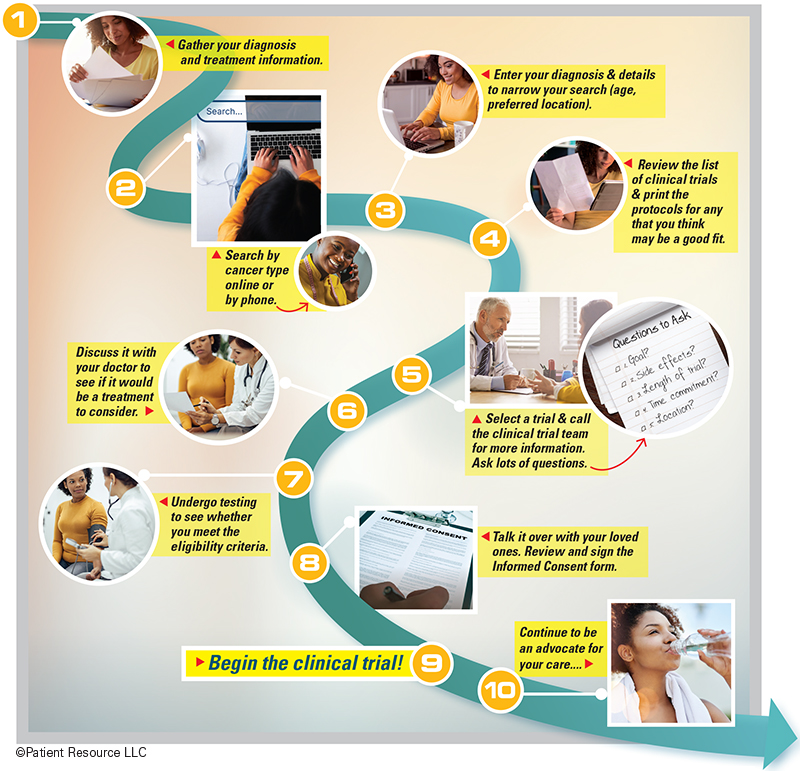
When looking for a clinical trial, there are some specific steps that you must take. Start by having a conversation with your medical team so you understand the types of trials they may be considering and then become an active part of your treatment team by joining the search. Follow the path outlined on this page to help guide your efforts and use the resources below to help you connect with advocacy groups and trial search databases.
Resources
- Cancer Support Community: www.cancersupportcommunity.org/find-clinical-trial, 888-793-9355
- Center for Information & Study on Clinical Research Participation: www.searchclinicaltrials.org, 617-725-2750
- ClinicalTrials.gov: www.clinicaltrials.gov
- Lazarex Cancer Foundation: www.lazarex.org, 877-866-9523, 925-820-4517
- National Cancer Institute: www.cancer.gov/clinicaltrials
- NCI Cancer Information Service: 800-422-6237
- WCG CenterWatch : www.centerwatch.com, 866-219-3440



