Understanding the Genomics and Genetics of Cancer
Treatment Options
Advances by cancer researchers who are focused on finding mutations that create and drive cancer are resulting in new types of drugs, innovative combination therapies and additional clinical trials. This personalized treatment strategy is offering people facing cancer more options and, ultimately, more hope for a prolonged life or even cure.
Your genomic testing results will indicate whether you have a specific mutation for which a treatment is available. Keep in mind that it is possible a mutation will not be detected, or that a mutation will be identified that does not have an approved treatment. In either of those cases, your doctor will discuss the other treatment options that are available. Those may include a clinical trial, as many are underway to continue finding effective treatments for additional mutations.
Should a mutation or abnormality be detected, your doctor will consider many factors before recommending a treatment plan tailored just for you. Those factors will include the tumor’s stage, grade and biomarker status; your general health; and your preferences concerning quality of life in regard to potential treatment side effects.
Take this opportunity to ask questions about the proposed plan. Before beginning any therapy, discuss the following:
- The side effects that may accompany each treatment option. Some may be mild while others may be severe. Ask your medical team what to watch for and what you should do if side effects occur. Some may prompt treatment to prevent more serious complications.
- The importance of medication adherence. Most cancer therapies are designed to maintain a specific level of drugs in your system for a certain time. To be fully effective, every dose must be taken exactly as prescribed, whether you receive it via IV, injection or pill form. Non-adherence can be serious, even life-threatening. Make sure you are capable of sticking to your medication and appointment schedules.
- How treatment effectiveness will be monitored. Your doctor will continually monitor your condition and make adjustments. Sometimes a therapy becomes less effective over time; other times, a new mutation may be discovered and a different therapy may offer more promise; or you may reach remission, among other things. When cancer cells stop responding to a drug, it is known as drug resistance, which is known to happen with some targeted therapies. In some cases, patients will have access to other targeted therapies that are designed for other substances within the cancer.
TYPES OF TREATMENT
Following are descriptions of therapies that are available to treat some mutations. These drugs can be given orally, intravenously or subcutaneously (see Figures 1 and 2).
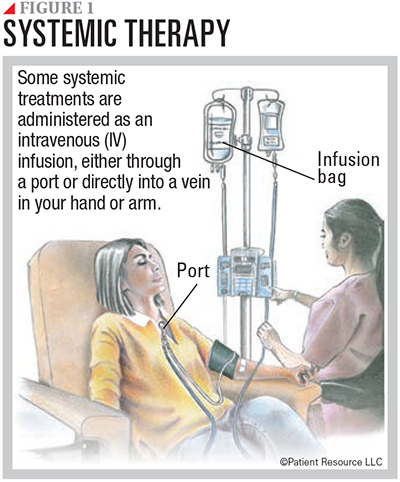
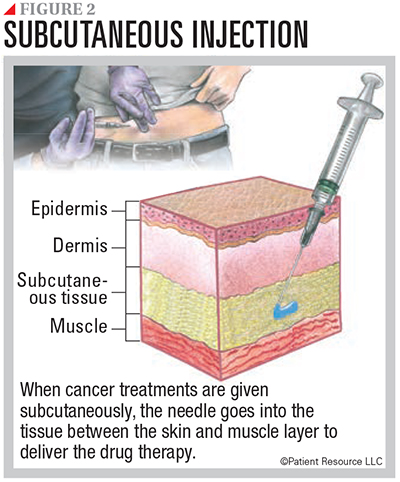
Targeted Therapy
This strategy uses the results from genomic testing to target specific genes, receptors, proteins, mutations, abnormalities or other factors involved in the development and support of the tumor. Because this therapy focuses on its target only, it typically does not damage nearby healthy cells, generally resulting in fewer side effects than traditional chemotherapy. They can attack cancer in the following ways:
- Preventing cancer cells from growing and from living longer than normal
- Blocking or stopping signals that help form blood vessels to the tumor
- Delivering cell-killing substances to cancer cells
- Causing cancer cell death
- Starving cancer of the hormones it needs to grow
Targeted therapies work in different ways and can be classified as small molecule drugs, angiogenesis inhibitors and monoclonal antibodies (mAbs). Some mAbs are also considered immunotherapy.
Small molecule drugs are able to get inside a cell and target its internal components. They can target the cancer’s gene expression, enzymes that affect the tumor or certain proteins.
- Apoptosis inducers cause cancer cells to undergo a process of controlled cell death called apoptosis.
- Gene expression modulators modify the function of proteins that play a role in controlling gene expression.
- Histone deacetylase (HDAC) inhibitors affect gene expression inside tumor cells.
- Kinase inhibitors block a type of enzyme known as a kinase. Certain kinases are more active in some types of cancer cell.
- Proteasome inhibitors target proteins to kill cancer cells.
- Selective inhibitors of nuclear export (SINE) enhance the anti-cancer activity of certain proteins in a cell.
- Signal transduction inhibitors block signals passed from one molecule to another inside a cell. Blocking these signals can affect many functions of the cell and may kill cancer cells.
Angiogenesis inhibitors block new blood vessel growth that feeds tumor cells. Tumors need a blood supply to survive and grow. Without it, the tumor cells cannot survive.
Monoclonal antibodies (mAbs) are proteins produced in a laboratory that are designed to attach to specific targets found on cancer cells. They can directly stop cancer cells from growing, cause them to self-destruct or deliver therapeutic agents directly to targeted cancer cells.
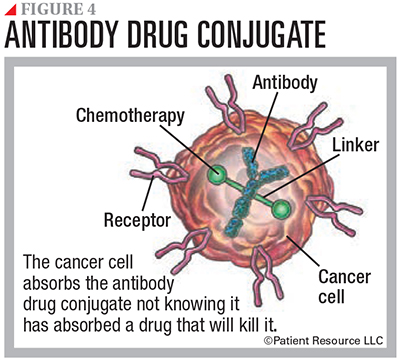
Many mAbs work by themselves. Others have a chemotherapy drug or a radioactive particle attached to them. Known as conjugated mAbs, they deliver treatment to the cancer cells. Types include radiolabeled antibodies, which deliver radioactive particles, and antibody drug conjugates, which deliver a chemotherapy drug (see Figure 4).
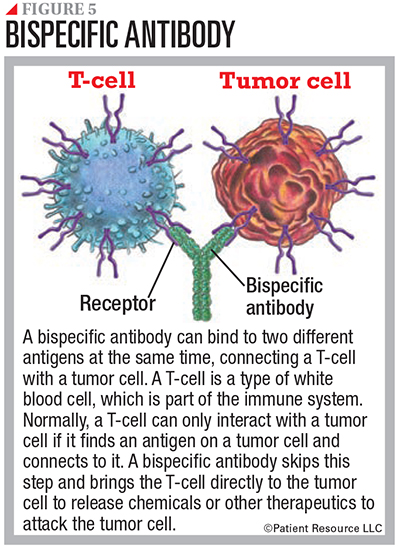
Another type, bispecific mAbs, are made up of two different mAbs that can attach to two different antigens at the same time.
Immunotherapy
A rapidly advancing and promising field in cancer treatment, immunotherapy recognizes and destroys cancer cells by harnessing the potential of the body’s own immune system. You may be a candidate for immunotherapy if your genomic testing results identify specific biomarkers such as PD-L1 expression, tumor mutational burden (TMB) and microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR).
Clinical trials are actively exploring the use of tumor-infiltrating lymphocytes (TILs) and chimeric antigen receptor T-cell (CAR-T) adoptive cell therapies. You may hear these cell-based therapy terms discussed and be a candidate for such immune system based treatments. The types of immunotherapy include immune checkpoint inhibitors and monoclonal antibodies.
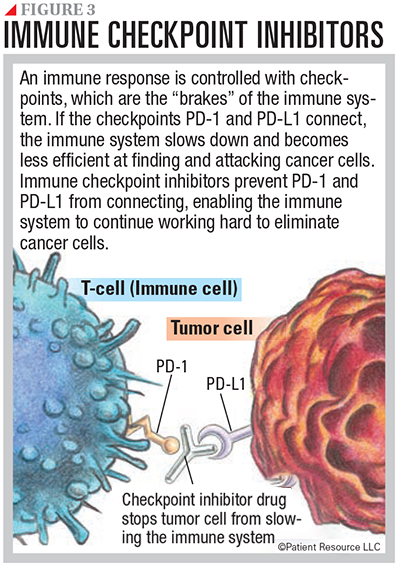
Immune checkpoint inhibitors prevent the immune response from slowing down, which allows the immune cells to continue fighting cancer. They also help the immune system to recognize cancer cells as foreign cells. Normally, the immune system is kept in check through biological checks and balances called checkpoints. Checkpoint-inhibiting drugs prevent connections between checkpoints so that the immune system does not slow down and can keep up its fight against cancer (see Figure 3).
Some immune checkpoint inhibitors are also approved as tumor-agnostic treatment, which means they are approved to treat any cancer that has the molecular alterations known as microsatellite instability-high (MSI-H), deficient mismatch repair (dMMR) or tumor mutational burden-high (TMB-H).
MSI-H describes cancer cells that have a greater-than-normal number of genetic markers called microsatellites, which are short, repeated sequences of DNA. Every time a cell reproduces itself, it makes a copy of its genes and DNA. During the process, errors in duplication can be made, much like a misspelled word. The body normally corrects the error, but sometimes it is not caught and fixed (dMMR). It then becomes a mutation that is reproduced in later versions of the cell.
Cancer cells that have large numbers of microsatellites may have defects in the ability to correct mistakes that occur when DNA is copied. When cancer cells have this feature, they are more sensitive to destruction by immune checkpoint inhibitors. MSI is also tested to determine which tumors may have developed because of a deficiency in correcting cell division errors.
TMB measures the number of mutations within a tumor to predict a patient’s response to immune checkpoint inhibitor treatment. TMB-H describes cancer cells that have a high number of gene mutations.
Monoclonal antibodies (mAbs) are antibodies made in a laboratory that target specific tumor antigens. Some mAbs mark cancer cells so that the immune system will better recognize and destroy them. Other mAbs bring T-cells, a type of white blood cell, close to cancer cells, helping the immune cells kill the cancer cells. They can also carry cancer drugs, radiation particles or laboratory-made cytokines (proteins that enable cells to send messages to each other) directly to cancer cells.
When used as an immunotherapy, a bispecific mAb engages and activates immune cells, such as T-cells, to attack a tumor, block dual signaling pathways, block immune checkpoints, or form a way to replace a missing functional protein (see Figure 5). These should not be confused with mAbs used as targeted therapy, which directly attack certain components in or on cancer cells.
Hormone Therapy
This treatment targets specific hormones and can be used to block the body’s ability to produce them and interfere with how they behave in the body.
Chemotherapy
Your doctor may use your genomic test results to see whether chemotherapy may be an effective treatment option for you.



