Prostate Cancer
Genomic & Genetic Testing
Cancer forms when one or more normal genes change, or mutate, and this leads to disruption of the controls on growth and mobility that maintain structure and function of normal cells. Cancer is ultimately a disease of our genes, which are pieces of DNA — the information plan for the growth and control of cells. Because several genes have been associated with the development of prostate cancer, one key to understanding and treating it is through genomic testing.
Mutations are generally described as one of two types. They may be inherited from your parents or occur during a person’s lifetime from environmental factors, such as tobacco use, ultraviolet radiation, viruses, age or random mistakes in replication of DNA. Your doctor may use specialized testing known as genomic testing (molecular testing) or genetic testing to determine whether you have mutations - either in your normal cells (this is called germline testing) or in your cancer cells.
Genomic Testing
This type of testing involves testing for mutations in the DNA of your cancer cells. Such testing is typically performed as part of the evaluation process to detect biomarkers, which are substances such as genes or molecules that can be measured in the blood, urine or other body fluids or tissues. Such markers may provide information that helps your doctor make a diagnosis or plan treatment based on the likely aggressiveness of your cancer, which is the likelihood that your cancer will be resistant to treatment or spread (metastasize) to other areas of the body. Such tests help determine whether your cancer is at high or low risk of spread or return despite standard treatment, and this may impact timing and type of treatments recommended. These tests are performed on a sample of cancerous tissue taken from a biopsy or surgical specimen.
Genomic testing may also be used to identify whether your cancer has mutations for which targeted therapies are available. Your doctor may look for mutations in the BRCA1, BRCA2 and other genes.
Genetic Testing
Genetics is the study of genes and the passing of genetic information and traits from parents to children (heredity). Some cases of prostate cancer are inherited, and abnormalities influencing such inheritance can be detected in normal cells (blood or cheek swab specimen). Finding mutations in some genes may indicate an increased risk of developing cancer. Just because someone tests positive for a genetic mutation does not mean they will develop cancer. It just means the risk is higher and additional screening may be needed.
Prostate cancer can run in families. If you have a family history of a particular type of cancer, you may consider genetic testing to find out whether you carry the gene or genes associated with familial risk of cancer. Some of the inherited mutated genes that have been associated with prostate cancer include:
- Hereditary Breast and Ovarian Cancer (HBOC) Syndrome associated with inherited mutations in BRCA1 and BRCA2 genes
- PALB2, RAD51D, RNASEL (formerly HPC1) and HOXB13 gene mutations
- ATM and CHEK2 mutations
- Mutations in the MLHL, MSH2, MSH6, PMS2 and EPCAM genes, which cause Lynch Syndrome (hereditary nonpolyposis colorectal cancer)
In addition, characteristics in either your, or your family, history may increase the risk of familial cancer, such as:
- Family history of breast, colon, ovarian, pancreatic or prostate cancer
- An initial diagnosis of high-risk, regional or metastatic prostate cancer
- Ashkenazi Jewish ancestry
- Any other cancer diagnosis
Knowing Your Family History
Because genetic mutations can be passed down through families, knowing the health history of your family could offer valuable insights. Preventing or detecting a cancer early offers the best chance of a successful treatment outcome. This information could help your family members be screened and monitored closely if they have a gene mutation associated with cancer.
Interpreting the results of genetic testing can be challenging, so talking with a genetic counselor is recommended. Special training enables a genetic counselor to guide you and your family members before and after you have genetic testing. Ask a member of your health care team for a referral.
Gene Mutations
Genes can mutate in a variety of ways that can increase a person’s risk of developing cancer. Some mutations that can occur in genes include the following.
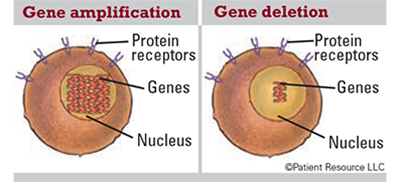
Gene amplification: An increase in the number of copies of a gene, which is common in cancer cells. Some amplified genes may cause cancer cells to grow or become resistant to anti-cancer drugs.
Gene deletion: The loss of all or part of a gene.
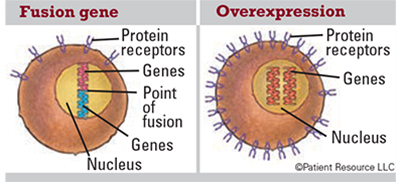
Fusion gene: A gene made by joining parts of two different genes. Fusion genes, and the fusion proteins that come from them, may be made in the body when part of the DNA from one chromosome moves to another chromosome. Fusion proteins produced by this change may lead to the development of some types of cancer.
Overexpression: Too many copies of a protein or other substance, which may play a role in cancer development.
Rearrangement: A mutation that occurs in chromosomes where portions of the chromosome are not in order, which creates a new gene (not shown).